Eye disease
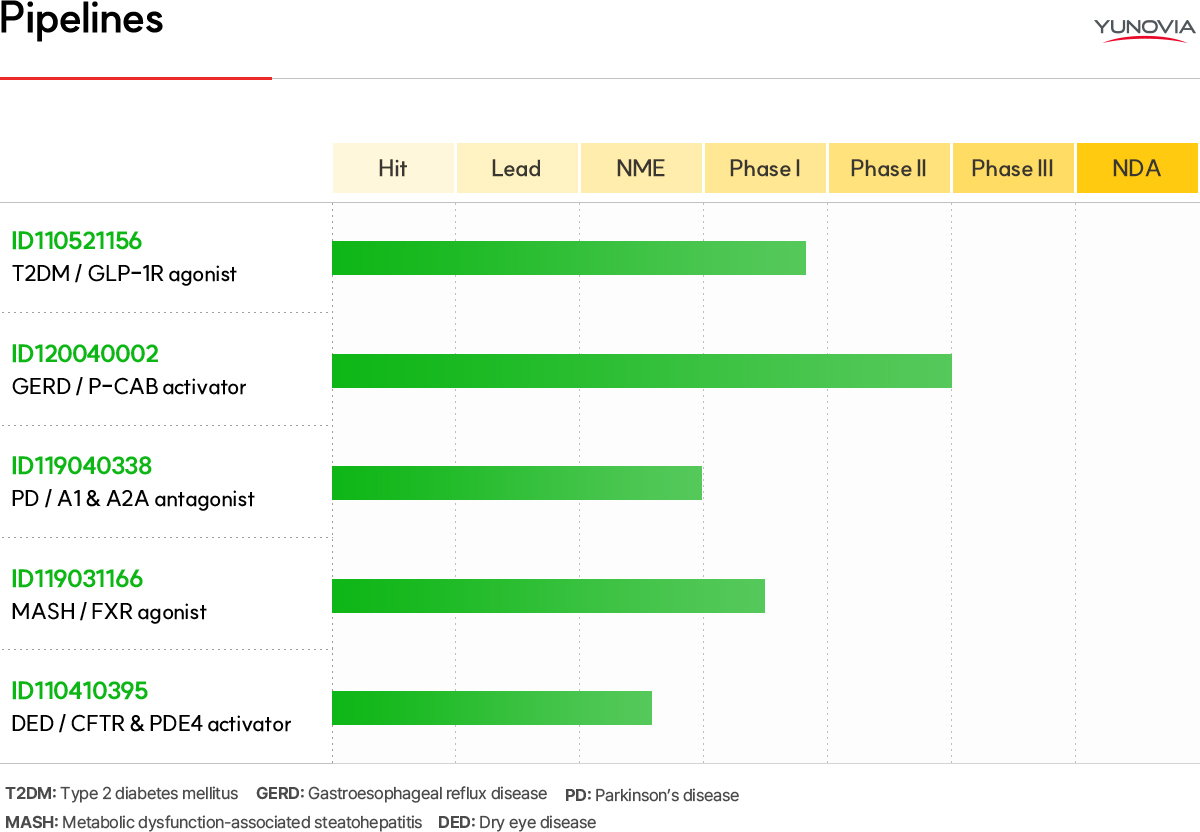
ID110410395 (CFTR activator/PDE4 inhibitor) Dry eye disease treatment
The dry eye disease treatment market holds promise, boasting an impressive average annual growth rate of 11.3%. Projections indicate that it will attain a substantial scale of $10 billion by 2028 across seven major countries. However, the current treatments face significant unmet needs due to adverse effects such as eye irritation, high dosing frequency, limited indications, and suboptimal effectiveness. To address this unmet need, there is a surge in the development of drugs with new mechanism of action.
Notably, ID110410395, functioning as a CFTR* activator and PDE4** inhibitor, is anticipated to effectively alleviate dry eye disease symptoms by reinstating tear film homeostasis through augmented tear secretion and the suppression of inflammatory expression. ID110410395, being a first-in-class drug, has demonstrated tear secretion and anti-inflammatory effects in both in vitro and in vivo disease models. The long half-life in the eye fosters expectations for enhanced patient convenience by reducing dosing frequency.
*CFTR: cystic fibrosis transmembrane conductance regulator
**PDE4: phosphodiesterase-4
