Liver disease
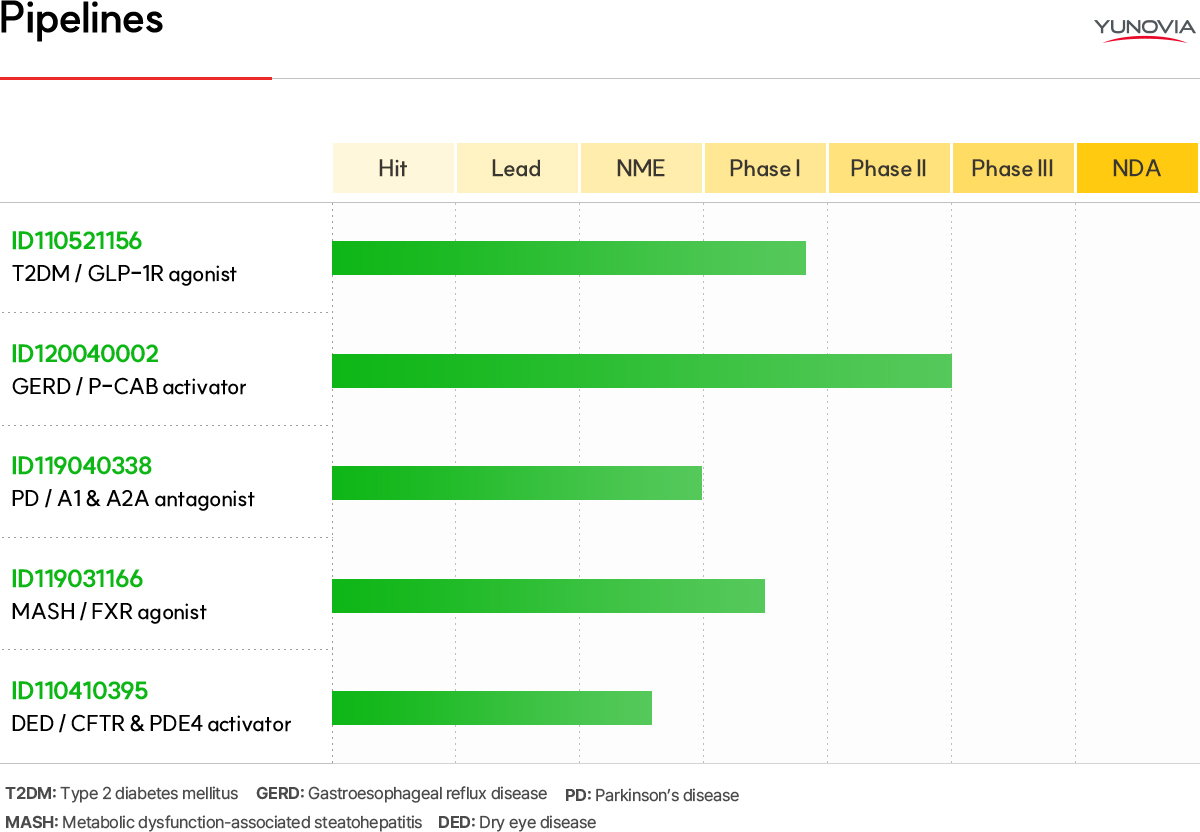
ID119031166 (FXR agonist) Metabolic dysfunction-associated steatohepatitis (MASH) treatment
Metabolic dysfunction-associated steatohepatitis (MASH, formerly NASH) stands out as a prevalent chronic liver disorder, characterized by the accumulation of neutral fat between liver cells, independent of alcohol consumption. It manifests symptoms of fatty liver and inflammatory signs of hepatocyte necrosis, potentially leading to Liver Cirrhosis and hepatocellular carcinoma. Current estimates suggest that approximately 1.5% to 6.5% of adults in the United States have MASH (ref. NIDDK). The market, projected to reach up to $7.1 billion by 2031 in major seven countries, including the United States, underscores a significant global demand for novel therapeutic interventions. However, there is still a lack of FDA-approved drugs for MASH treatment.
ID119031166 is a Best-in-Class drug, demonstrating superior FXR receptor activity and selectivity compared to endogenous ligands in in vitro studies. It has been verified to exhibit therapeutic effects on MASH and inhibition of liver fibrosis in MASH animal models. Additionally, it is anticipated to not engage in the activation of TGR5 (Takeda G-Protein Receptor 5), the mechanism responsible for itching side effects induced by the FXR Agonist OCA (Obeticholic acid). This raises expectations for potential improvements in side effects.
We have completed recruitment for our Phase 1 clinical trial in healthy adults in the US and plan to move forward with a Phase 2 trial based on the results.
