Diabetes
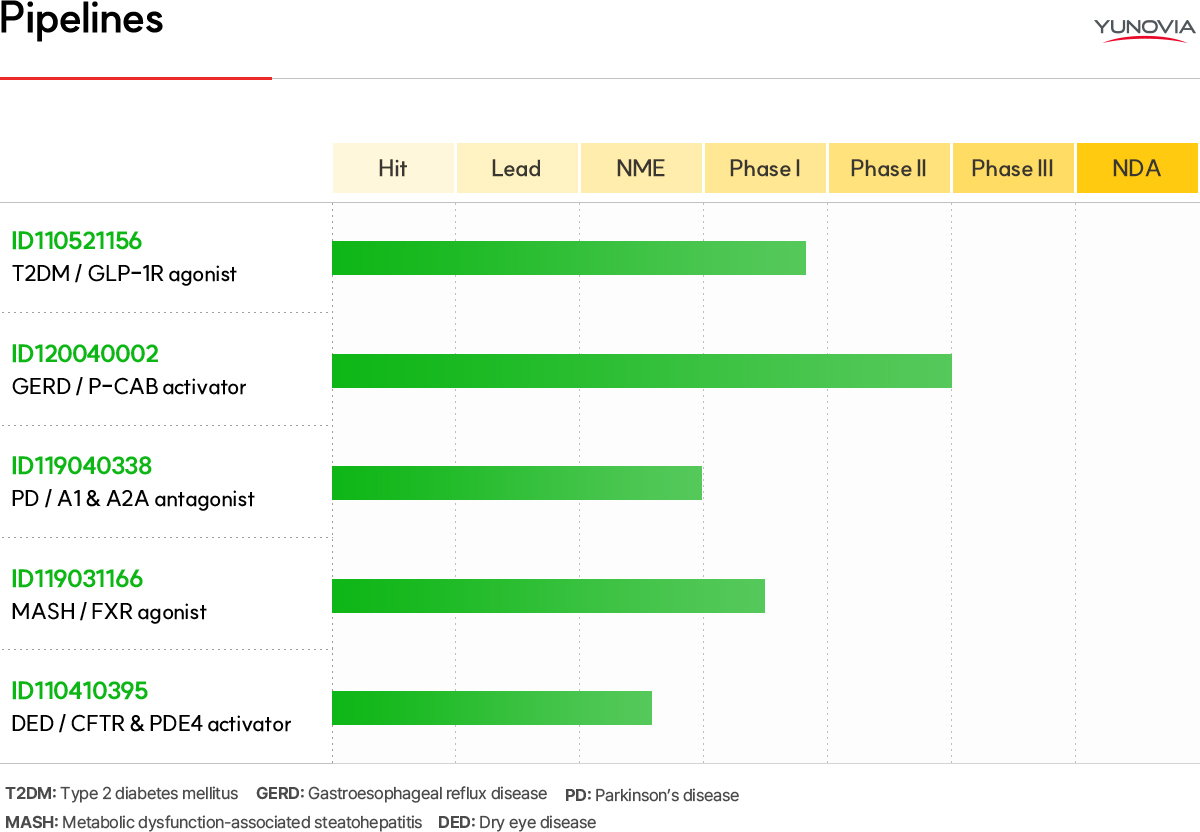
ID110521156 (GLP-1R agonist) Type 2 diabetes mellitus / Obesity treatment
The type 2 diabetes mellitus market is expected to be valued at $41B in 2022, with a CAGR of 6% (2022-2040), and the GLP-1 RA market is expected to be valued at $12.9B in 2022, with a CAGR of 27% (2022-2040), accounting for 29% of the total type 2 diabetes market.
Type 2 diabetes mellitus is a chronic disease characterized by insulin resistance and pancreatic β-cell dysfunction. Among various therapeutic agents, drug agonists with actions similar to glucagon-like peptide 1 (GLP-1), a hormone in the body that increases insulin secretion, have garnered significant attention.
ID110521156 is a small molecule GLP-1 receptor agonist being developed as an orally administered therapeutic drug. Its mechanism of action involves increasing intracellular cAMP levels by activating the Gα subunit. GLP-1 receptor agonist can reduce the risk of hypoglycemic shock through glucose-dependent insulin secretion. As a small molecule compound, it is expected to address the drawbacks of existing injectable peptide drugs (such as side effects).
GLP-1 is a peptide belonging to the incretin family, released approximately 15 minutes after food consumption. It stimulates insulin release, regulating blood sugar levels and diminishing stomach acid production. Notably, it plays a crucial role in weight loss by decreasing gastrointestinal motility, delaying food passage, and inducing a sense of satiety in the brain. Due to these mechanisms, GLP-1 is gaining significant attention as a potential treatment for obesity.
ID110521156 has devised a comprehensive development plan for both obesity and diabetes adaptations. In line with this strategy, the drug is set to undergo parallel development, including clinical trials focused on diabetes and obesity. The current research landscape includes the investigation of GLP-1 agonist drugs, both those already on the market and those in various stages of study, to explore their potential in addressing obesity. This dual approach underscores the versatility and potential impact of GLP-1 in addressing both diabetes and obesity.
Phase 1 Single Ascending Dose (SAD) dosing has been started in November 2023 and treatments for last cohort were finished in July 2024 currently. The MFDS IND for the phase 1 Multiple Ascending Dose (MAD) study was filed in June 2024. It is expected that first dosing is started in 4Q and the MAD study is finalized in 2025.